History of Pacemakers
![]()
1700s
De facto cardiac
electrostimulation began in the mid-eighteenth century with the use of currents
from the Leyden jar or Voltaic Pile (Allessandra Volta, 1799) to stimulate
cardiac nerves and muscles in animals and to attempt resuscitation of intact
dead animals.[6] Dr. William Hawes in London established The Humane Society of
London in 1774 AD - A fraternity devoted to salvaging persons seemingly dead -
motivated by similar such society in Paris. Later on it became The Royal Humane
Society of London. Squires, Henley and Fothergill suggested Electrostimulation
for resuscitation in a number of communications to the Society between 1774 and
1748.[7] Such an incidence is described by Charles Kite in his “An Essay upon
the Recovery of the Apparently Dead.” (
“With the consent of the parents, very humanely tried the effects of
electricity. Twenty minutes had at least elapsed before I could apply the
shock, which I gave to various, part of the body without any apparent success;
but at length, on transmitting a few shocks through thorax, I perceived a small
pulsation; soon after that child began to breath, through with great
difficulty. In about 10 minutes she vomited. A kind of stupor remained for some
days, but the child was restored to perfect health and spirits in about a
week.”[8]
|
|
|
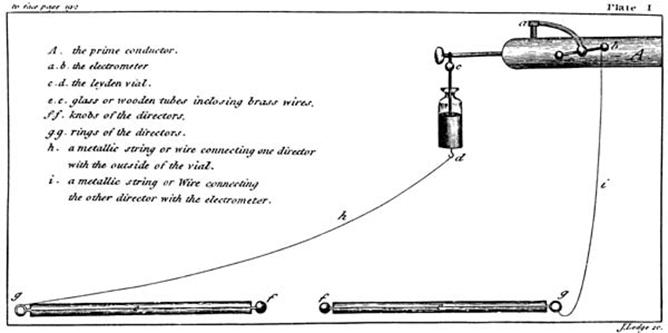
Apparatus as shown in Kite’s ‘An Essay upon the Recovery of the
Apparently Dead’ (1788) |
Charles Kite commented on this,
“Do (these incidences) not plainly point out that electricity is the most
powerful stimulus we can apply, and we not justified in assuming, that if it is
able so powerfully to excite the action of the external muscles, that it will
be capable of reproducing the motion of the heart which is infinitely more irritable,
and by that means accomplish our great desideratum, the renewal of the
circulation.”8
However, it is noteworthy that we have almost matching description of
resuscitation by electricity, this time by two Danish Scientist Herboldt and
Rasn (1796) in their small booklet “Life saving measures for drowning persons
and information of the best means by which they can be brought back to
life.”[9]
![]()
In 1802, Nysten used a human cadaver shortly following death by execution to demonstrate that the ability to reactivate the heart electrically was lost earlier for the left ventricle, later for the right ventricle, still later for the left atrium and last for right atrium.12 Later in the nineteenth century, Walshe (1862) and Duchenne (1870) advocated electrostimulation for cardiac standstill.[13],[14] During the same period, Althaus (1864) reported successful resuscitation of cardiac arrest victims by electrical currents applied through transthoracic needle.[15]
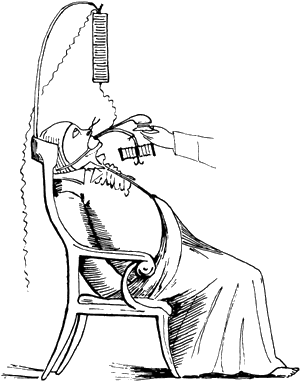
Dr.DeSanctis used “Re-animation Chair.”(Fig.2) as described by Richmond Reece in his The Medical Guide (1820). It had 3 pertinent features: a bellows to give forced ventilation, a metallic tube to be inserted into the esophagus and a voltaic pile attached at one pole to the esophageal tube and at the other to an electrode. The electrode was to be successively touched to “the regions of the heart, the diaphragm and the stomach…”[10] These reports may have lead John Hunter to recommend in 1776 that electrostimulation be tried as the as the resort in the resuscitation of drawing victims.[11]
|
|
|
The Re-animation chair of Dr. DeSanctis (1820)
|
By the early 1800s, there appeared a renewal of interest in acupuncture in Europe that had been introduced into Europe in the second half of the eighteenth century by Jesuit missionaries. In 1825, Sarlandie`re was the first to apply an electric (galvanic) current to thin metal needle electrodes (derived from acupuncture needles) thus creating electropuncture for the application of current to specific points on or in the body. Electropuncture soon became the accepted method of stimulating muscles, nerves, or organs beneath the skin . Electropuncture of the heart was first attempted by Krimer in 1828 without recorded success.
|
|
|
Krimer's Electropuncture of the Heart (1828)
|
This technique was then abandoned for several decades. Meanwhile, W. Morton successfully introduced the use of ether as an anesthetic in 1846. Eventually, chloroform was found to be more suitable although cardiac arrest was a frequent complication of chloroform anesthesia in those early days. In 1871, Steiner overanesthetized horses, dogs, cats, and rabbits to produce cardiac arrest. He reported successfully applying an intermittent galvanic current to a percutaneous needle in the heart to evoke rhythmic contractions. Terms such as galvano and farado puncture soon started to appear in the literature .
In 1882, Von Ziemssen described a case of a 42-year-old lady named Catherina
Sarafin who had a huge defect in the anterior left chest wall following
resection of an enchondroma. The heart was covered by a thin layer of skin and
was visible and palpable. Von Ziemssen noted that application of electrodes to
the heart resulted in rhythmic stimulation only it the rate of stimulation was
greater than that of the spontaneous heart rate. Slower stimulation produced
erratic and sometimes slower heart rate. He also noted while placing his
electrodes that the most sensitive area for stimulation was in the region of
atrioventricular groove. [16] Interestingly this observation was made more than
a decade before the description by Kent and His of the location of the
atrioventricular node and bundle of His, respectively [17],[18]
In 1899, Prevost and Battelli demonstrated that electrical currents could cause
ventricular fibrillation that often could be reversed by another powerful
stimulus of either alternating or direct current.[19] Robinvitch in a series of
reports from 1907 through 1909 confirmed this work and designed the first
portable electrical resuscitative apparatus for ambulances.[20] MacWilliam, in
many publications beginning in 1899 and extending to World War I, further
elucidated the pathophysiology of ventricular fibrillation and described
deterioration of cardiac pump function by tachyarrhythmias as well as
bradarrythmias. [21]
Interestingly, the experimental and clinical experiences just described did not
lead to immediate clinical trials of either cardiac pacing or electrical
defibrillation. It was not until the efforts of Kounwenhowen (1932) and Beck
(1947) that electrical defibrillation became widely used clinically.[22],[23]
Studies in Europe by Marmrostein in 1927, using both transvenous and
transthoracic electrodes to pace right atrium, right ventricle and left
ventricle in dogs were essentially unnoticed in United States.24 This is
evident from the reports in 1950 by Wilfred Bigelow, John A. Callaghan and Jack
Hopps who independently described similar studies using transcutaneous
electrodes to pace right atrium of dogs.[25] In 1949 during an experimental
operation, a dog’s heart suddenly stopped at 21 ºC. “Out of interest and desperation,”
recalls Dr.Bigelow, “I gave the left ventricle a good poke with a probe I was
holding.”[25]All the four chambers of heart responded to it and further pokes
clearly indicated that the heart was beating normally with good blood pressure.
He immediately discussed it with Dr. Callaghan. Using dogs and rabbits, they
again collected the data, studying the most effective and safe electric current
and made movies of their key experiments to present before the Annual Surgical
Congress of the American College of Surgeon’s meeting at Boston in October
1950. As Dr.Callaghan had done a “Lion’s share of work, particularly in the
normal body temperature studies, “he made a ten minutes presentation.” Which
was one of the scientific highlights of the day with great interest from the
media.”[25] chuckles Dr.Bigelow. Co-incidentally their Co-worker Jack Hopps
also later became a pacemaker recipient.
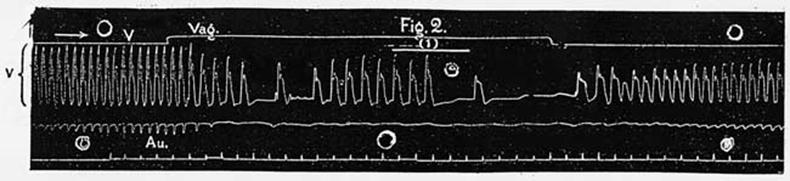
Electrical Stimulation of the Heart in Man by McWilliam – 1889
“The electrodes should be of considerable extent (for example, large sponge electrodes), and they and the skin should be well moistened with salt solution. The shocks employed should be strong, sufficient to excite powerful contraction in the voluntary muscles. Such a method, it seems to me, is the only rational and effective one for stimulating by direct means the action of a heart which has been suddenly enfeebled or arrested in diastole by causes of a temporary and transient character.”
|
|
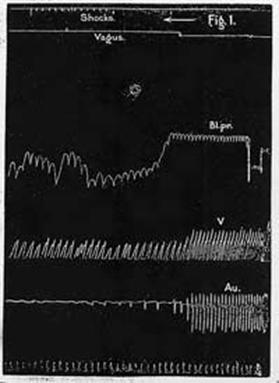
Figure 1. Cat's heart. The heart was depressed by
vagus stimulation at the point indicated in the second tracing from the top
to the figure. A marked fall of blood-pressure ensued. Then single induction
shocks were sent into the heart. These shocks cause a more rapid and more
effective series of ventricular beats. The lowest tracing marks time in
half-seconds.
|
|
|
|
Figure
2. Cat's heart. The lowest line indicates half-seconds. A periodic series
of (eight) induction shocks was applied to the ventricles. The resulting
group of beats is marked (1). The individual beats are much improved in
strength as compared with the spontaneous beats occurring before and after,
marked (2). |
McWilliam JA. Electrical stimulation of the heart in man. Brit Med J 1889;1:348-350.
![]()
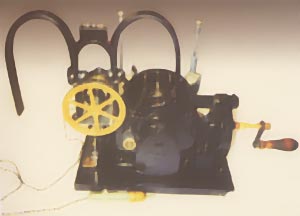
Hyman Pacemaker
|
In 1930 Albert S. Hyman. with
his brother, an engineer, developed and patented the "artificial
pacemaker" operated by a hand crank and spring motor which turned a
magneto (DC current generator) to supply the electricity. The device was used
in the |
 |
|
|
In
1932, Albert Hyman developed a machine for controlled repetitive
electrostimulation of heart and named his device the “artificial cardiac
pacemaker.” [26] (Fig.3) In Hyman’s Words: “Finally on April 6,1930, I received Grant No. 30-2 from the Witkin Foundation to explore the possibility of developing a practical machine, to be used as an artificial pacemaker in experimental animals. Reduced to its simplest blueprint from such an apparatus would include, |
i. A small source of electric current, i.e. a common flashlight battery.
ii. An interrupter mechanism
iii. A timing device
iv. A method of regulating the duration of the injected current; and
v. A suitable insulated needle to carry the current only to the right atrium of
heart.
The instrument would, of course be easily portable and small enough to fit into
a doctor’s bag.
The next 10 months were devoted to the assembly of such an apparatus…
By March 1, 1932 the artificial pacemaker has been used 43 times with a
successful outcome in 14 cases.”[27]
 |
During his
active promulgation of the artificial pacemaker (a term he coined)
Albert S. Hyman claimed to have designed and had constructed several
different models, although only a single model was ever described,
or a photograph of which (Hyman AS. Resuscitation of the stopped
heart by intracardial therapy. II Experimental use of an artificial
pacemaker. Arch Intern Med 1932; 50:283-305) published in the
medical literature. A photograph of another model without any
description had been found. It was that of a clearly portable unit
which presumably had been manufactured by Siemens, the parent
company of Adlanco, in their German plant. It had been long assumed
that this unit was manufactured during the late 1930s in order to
test the potential for greater commercial exploitation, but had not
been continued, as a consultant to |
Siemens had determined that the
pacemaker would not accomplish its intended function and that all actual models
built had been destroyed during World War II. Surprisingly, a copy of Popular
Science of October 1933 was found by chance, containing a single page
article concerning the Hyman pacemaker with a photograph of Hyman's engineer
brother, Charles, "resuscitating" a young man. In the background is
the pacemaker thought to have been manufactured only years later.
From this article the date of manufacture must
have been sometime during 1932-1933. The one other photograph extant is so
clear that the labels on the dials can be read and interpreted as they were in
an article about the reconstruction of the Hyman I pacemaker
by the NASPE history project (Furman S, Jeffrey K, Szarka G. The Mysterious
Fate of Hyman's Pacemaker. PACE 2001;24:1126-1137). With the knowledge
gained from the reconstruction of the earlier device and the information
concerning the function of each of the dials in the "orphan"
photograph, the second device was also reconstructed as a working model. Unlike
the earlier model which is hardly portable, weighing XX kilograms, the
"portable Hyman II weighs YY kilograms, has a carrying handle and was
intended to be brought to the site of use. The size of the device was estimated
from the size of the handle, obviously intended to be operated by a human hand.
Both
models are "working" in the sense that movable parts do move and for
the Hyman II the small electrical output Hyman intended, incapable of causing a
cardiac response, is emitted. From analysis of the electrical outputs of both
Hyman devices it should not be expected that either would have been capable of
resuscitating any asystolic mammalian or human heart.
The
photograph shown is the original and may be compared with the reconstructed
device. Many years later, after the invention of pacing, as it is known today,
Albert S. Hyman was interviewed by historian David Schechter and quoted in his
book (
Both these devices are the first known to bear the name artificial pacemaker and to have been specifically built and designed for cardiac resuscitation during asystole.
|
|
|
|
Popular Science, October 1933 |
The only photograph existing of the Hyman Portable Pacemaker |
![]()
|
|
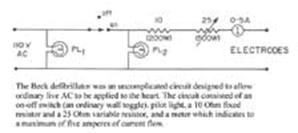
If ventricular fibrillation develops in hearts that are relatively normal, especially during operations, defibrillation may be life saving. During the period before regular rhythm is restored the heart must be exposed and rhythmically massaged in the interval. The method consists of the injection of 5cc. of 2 per cent procaine hydrochloride into the right side of the heart followed by a brief period of cardiac massage to distribute the drug throughout the myocardial bed. The heart is then placed between two large electrodes and ordinary 110 volt alternating current with 1.5 amperes is momentarily impressed through the heart between the electrodes.Usually a series of such shocks is necessary to accomplish defibrillation. If the treatment is successful, the ventricles cease fibrillating and remain in standstill momentarily before a supraventricular rhythm is established. Massage is again continued until the vigor of the heart beat is sufficient to empty the cardiac cavities of blood. Defibrillation of the human ventricles at the operating table has been performed five times at this hospital in the past, but all patients have subsequently died |
without regaining consciousness. We are reporting the first case with complete recovery after prolonged ventricular fibrillation. We present it with the hope that, following Beck's suggestion, operating rooms will be equipped to handle cases of sudden ventricular fibrillation and that personnel will be trained in the method. Speed and precision in the technique are important.
A
boy aged 14, was admitted to Dr. Claude Beck's service of this hospital for
sternal resection because of severe congenital funnel chest. …During the
closure of the wound in the chest, the pulse suddenly stopped and blood
pressure sounds could not be heard. The patient was apparently dead. The wound
was reopened and cardiac massage immediately was initiated…. The mechanical
respirator was attached to the intratracheal tube and cardiac massage
continuously given for the next thirty-five minutes, at the end of which time
the electrocardiogram was characteristic of ventricular fibrillation. Another
record ten minutes later was recorded just prior to the first electric shock
applied directly to the heart. This record, as well as the tracing recorded
immediately after the first shock, also showed ventricular fibrillation. Since
the first shock was unsuccessful, procaine hydrochloride was injected into the
right auricle, the heart massaged and a second series of shocks given. The
heart then was in standstill.
Almost
immediately, however, feeble, regular and fairly rapid cardiac contractions
were seen, but massage was continued for five more minutes, at which time it
was obvious that contractions were coordinated and fairly vigorous, though
still fast. An electrocardiogram taken at this time revealed a supraventricular
tachycardia at a rate of 175. From this point on the heart gradually increased
in vigor and brachial sounds were heard at 50 mm. of mercury.
|
|
|
|
|
Front
Panel (Exterior) |
Fixed
Resistor (Interior) |
Variable
Resistor |
|
|
|
|
|
The
Circuit |
Figure 2 – (a) first recorded
electrocardiogram at operation showing coarse ventricular fibrillation. |
Figure 3 – Electrocardiogram two months
after operation; normal record. T wave in lead II is now upright. |
Beck
CS, Pritchard WH, Feil HS. Ventricular Fibrillation of Long Duration Abolished
by Electric Shock. J Amer Med Assoc. 1947; 135(15):985-986.
Geddes
LA, Hamlin R. The First Human Heart Defibrillator. Amer J Cardiol 1983;
52:403-405
All
photos of the Beck Defibrillator are courtesy of The Smithsonian Institution
![]()
Long
Term Ambulatory Exteriorized Transvenous Pacing
HN, a 67-year-old man with 2:1
and complete heart block had had syncopal episodes for several years. He
underwent insertion of a transvenous lead via cephalic vein cut down, on May
19, 1959, was hospital discharged on June 23, 1959 and lived at home as an
ambulatory outpatient until November 1962. During that time he was paced with
the newly available, battery operated model 902M (Atronic Products, Inc.
|
|
|
|
Atronic Products Model 902M
|
New
York Mirror June 23, 1959 |
|
|
|
|
New
York Daily News June 23, 1959 |
New York Times June 23, 1959 |
Schwedel JB, Furman S, Escher DJW. Use of an Intracardiac Pacemaker in the treatment of Stokes-Adams Seizures. Prog Cardiovasc Dis 1960;3:170-177.
First Working Pacemaker Implant
were still in use in |
|
||
|
|
|||
|
Dr.
Elmqvist was able to provide an implantable pulse generator powered by two
rechargeable nickel-cadmium batteries, each delivering 50 microampere/hours.
Recharging was accomplished by a 150 kHz current generated by an external 220
volt unit. The current was transmitted by induction from an external flexible
coil 25 cm in diameter placed on the skin over the pacemaker, to a coil 50 mm
in diameter within the implanted generator. The pacemaker required charging
once a week for 12 hours. The cylindrical unipolar asynchronous implantable
generator consisted of the nickel-cadmium batteries, the electronic circuit
and the recharging antenna, all encapsulated in epoxy. It was 52.5 mm in
diameter, 17.5 mm thick and weighed 64.3 grams. The lead had a braided nylon
core surrounded by four flat stainless steel bands insulated by a
polyethylene coating. The stimulating electrode was a platinum disc 9 mm in
diameter, to be sutured to the epicardium through two small holes. The
cathodal stimulating surface area was 63.6 square mm, the anode was a metal
ring 10 mm wide on the pacemaker's edge. The ring was not completely
circumferential to avoid interruption of the magnetic field created by the
charging current. Dr. Robert Rubio implanted this unit on February 3, 1960 at the CASMU Clinic of Montevideo, Uruguay. The epicardial electrode was sutured to the left ventricular surface and the pulse generator was placed in the abdominal wall. Her early course was of an increased exercise tolerance and the absence of Adams-Stokes seizures. Infection developed in the thoracic incision and she died of sepsis on October 20, 1960, 9 ½ months after pacemaker implantation.
Fiandra
O. The First Pacemaker Implant in |
|||
|
|
CATALOGUE # : 850610
# PARTS: 003
Type: OPEN CHEST
Model: S-D4
Serial #: 11/PROTOTYPE
Manufacturer: NRC
Address #:OTTAWA ON CAN
Year Code: X From Date: 1950 To
Date: 1955
Article Eng Description:
STIMULATOR & DEFIBRILLATOR
Comments:
BREAKDOWN: 1 Stimulator-difibrillator; 2 Foot Padel Micro Switch; 3 Two
Electrdes/Foot Pedal allows surgeon to control flow of electricity while
positioning electrodesby hand/electrodes placed on either side of the heart for
open chest model on either side of chest wall in later closed chest
mdels/outcome of hypothermia experiments by hopps and bigelow/appeared on
Clarence Walton Lillehei: The father of Cardiac Surgery
|
|
Development of surgery of heart
has been one of the most dramatic phenomena of the 20th century. Indeed,
C.Walten Lillehei played a heroic character on stage for more than 40 years.
Dr. Denton Cooley describes him as “Role model for investigators.”32 One such
example is found from his speech delivered at the Bakken Library and |
|
|
|
Radiofrequency Epicardial Pacing
|
|
|
Soon
after the introduction of cardiac pacing with exteriorized myocardial
electrodes it was apparent that peri-electrode infections would limit long-term
pacing. To avoid this complication there was developed and introduced
clinically in 1958-59 a totally implantable battery-powered pacemaker and a
radiofrequency (RF) stimulator inductively coupled through intact skin (Mauro’s
technique) to pace the heart. After several years, the addition of a
long-lasting lithium battery and the ability to externally program the pacing
parameters favored the sole use of a totally implantable cardiac pacemaker.
The
use of the rf induction method for stimulating excitable tissue was expanded to
pace the diaphragm, contract the paralyzed urinary bladder and muscle
sphincters, and enhance hearing (cochlear implant).
Glenn
WWL, Mauro A, Longo E, Lavietes PH, MacKay FJ The Radiofrequency Cardiac
Pacemaker. Remote stimulation of the heart by radiofrequency transmission.
Clinical application to a patient with Stoke-Adams Syndrome. New Engl J Med
1959:262;948-951
In 1958, in
|
|
|
||||||||||||||||||||||||||||||||||||||||
At that time it was thought that the pulse
generator should deliver impulses of about 2 volts and an impulse period of
about 1.5 ms at a rate of 70-80 per minute, using as little energy as possible.
Fortunately, silicon transistors had just appeared on the market.
Among the many types of primary cells, the
Ruben-Mallory cells with zinc as the anode and mercuric-oxide as the
depolarizer were a possible choice. I had already had experience with the
mercury cells … their lifetime was short. Besides, I did not know what effect
the hydrogen gas development at the zinc anode would have on a cell
encapsulated in plastic. For that reason, nickel-cadmium rechargeable cells
were then chosen. Two cells of 60 mAh each were connected in series.
A coil with a diameter of about 50 mm was connected
to the cells via a silicon diode. The charging current came from a
line-connected vacuum tube radiofrequency generator with a frequency of 150
kHz. A large flexible coil in the output circuit of the generator could be
attached to the patient's abdomen with adhesive tape. Recharging was done
overnight … about once a month. The whole apparatus was encapsulated in epoxy
resin. The diameter was about 55 mm and the thickness about 16 mm.
The first apparatus had two electrode wires, each
consisting of a twined, stainless suture wire with polyethylene insulation sewn
in the myocardium … It soon appeared that the wire was unsuitable as an
electrode. The stimulation threshold increased and after only a few weeks, the
unit ceased to stimulate. The patient again went into heart block, but the
Adams-Stokes attacks did not recur.
American manufacturers were predicting a five year
lifetime for pacemakers with mercury-zinc batteries, and since we felt that rechargeable
units were cumbersome for patients, we also began to use mercury-zinc cells. I
am not convinced that it was right to give up the rechargeable cells since, at
that time, the quality of mercury cells was not high enough.
I must admit that I had regarded the pacemaker more or less as a technical curiosity. It has, therefore, been gratifying for me to follow its enormous success, and to have taken part in its development.
Elmqvist R. Review of Early Pacemaker Development.
PACE 1978; 1:535-536.
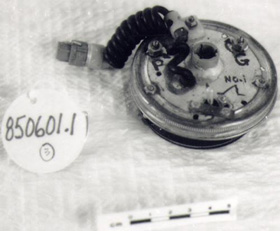
Canadian
Medical Innovation, Developed and Manufactured at National Research Council,
Canada.
Example
of very early Cardiac Stimulator-Difibrillator. (First was built by Jack Hopps
C. 1950) Instrument used 60-Hz current at voltages up to 220V to arrest
fibrillation through the open chest. The shocks regulated and/or restarted the
beating of heart (stimulation), or were intended to instaneously stop and
restart beating of heart muscle.
Function: To stimulate a stopped heart to resume beating, and to regulate
heart-beat.
Significance to Technology:
Experimental pacemakers used to study the threshold of pacing, non-
implantable because researchers needed across to the controls to vary the
speed.
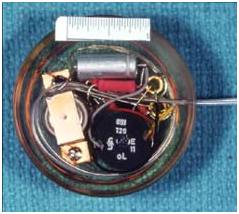
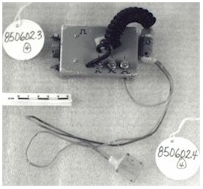
More
sophisticated version of round pacemakers 850601. Prototype variable speed
pacemakers for laboratory use to study the threshold of heart pacing, waveform
studies and pulse widths.
|
|
CATALOGUE # : 850601
# PARTS: 003
Type : NON-IMPLANTABLE/ROUND
Serial # : PROTOTYPE
Manufacturer : NRC
Address # :
Year Code : X From Date: 1965 To
Date: 1970
Article Eng Description:
PACEMAKER, CARDIAC
Comments : NO. BREAKDOWN:.1-.3
PACEMAKERS/
Used on dogs, plugged into an external opening in the back
of the animal, held in place with a silastic interface. At one time Dr. O.Z.
Ror had 6 dogs connected to external pacemakers at once in a long term study of
the efficiency of heart pacemakers.
Significance to
Part of NRC pacemaker program. 1st Pacemaker developed at NRC in
1950 by John Hopps was approximately 30CM long and several CM high & wide.
Significance to Technology:
Development of transistors & reliable batteries permitted design of
pacemaker of implantable size (1957). Pacemaker regarded as one of the greatest
Canadian engineering achievements of the 20th century.
 |
 |
 |
|
CATALOGUE # : 850602 Used on
dogs, connected to opening in back of animal & held in place with
silastic interface. At one time Dr. O.Z. Roy had 6 dogs connected to external
pacemakers at once in a long term study of the efficiency of heart
pacemakers. |
|
Reprinted courtesy of National Research Council of Canada |
Manufacturer : National Research Council of Oblong
type used in experimental program to deliver electrical stimulus to the heart,
in order to stimulate rhythmic heart beat. Tried
in animal experiment of biological energy supplies, in which metals in
contact with body fluids acted as a natural battery. |
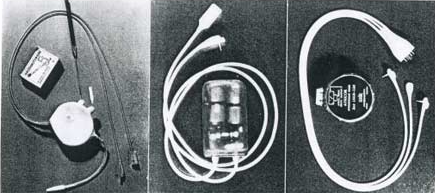
Transformer-Type Cardiac Pacemaker (Direct Induction Pacemaker)
|
|
This pacemaker consists of an external pulse generator (a), an external transmitting coil (b), an internal receiving coil (c), and myocardial electrode (d). Both the transmitting and receiving coils contain an iron-core strip, with which effective electromagnetic coupling between the coils is achieved, thus inducing stimulating pulses in the receiving coil without high frequency carrier waves.
From 1964 to 1968, the pacemaker was used in
fourteen patients at the University of Tokyo Hospital. The average period of
usage was forty months (two months to ten years and four months). Suma, K. et al: Direct induction pacemaker. Digest 6th Int Conf Med Elect Biol Eng (Tokyo) 1965; 96-97. |
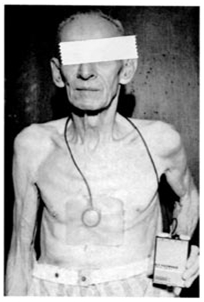
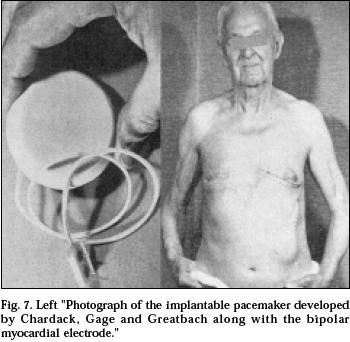
A Self-contained, Implantable Cardiac Pacemaker
|
|
After two years of experimental
work in the animal laboratory the patient shown in the photograph was referred
to me. 77 years old, he was in complete heart block. The operation was
completed in June 1960. His subsequent course was uneventful until he died
two years later of natural causes. He was the first patient whose heart block
was corrected successfully, on a long-term basis (years) by an implanted
pacemaker carrying its own power supply. The case was reported in 1960 and I
am gratified and grateful that the statements in the title of the report are
still valid. |
|
First Pacemaker Implantation in Germany
HJ Sykosch-Düsseldorf: Langenbecks Archiv für klinische Chirurgie, Band 308 (1964) 54. Inplantierbare Schrittmacher zur permanenten und intermittierenden Stimulation des Herzens.
|