The Body Bioelectric
The electrical activity of the body has been used for a long time for both diagnostic and monitoring purposes in medicine, largely in connection with the ‘excitable’ tissues. Examples include ECG, EMG, EEG. More recent developments have begun to look at the tissues which were not regarded as excitable, but in which, endogenous electrical activity has been demonstrated. The endogenous electrical activity of the body arises from a variety of sources, some of which are well documented whilst others remain more obscure in their origins & control mechanisms (Offner 1984, Leonesio and Chen 1987). The relationship between endogenous electrical activity (not exclusively potentials), injury & healing have been researched in several areas of clinical practice.
These investigations appear to follow three main themes:
- that the endogenous electrical activity of the body can be used as an indicator of a particular pathological process without necessarily attributing a cause/effect relationship. (Edelberg 1971, 1977 Marino et al. 1989, Woodrough et al. 1975).
- that the endogenous electrical activity of the body acts as an initiator, control mechanism or modulator of the post embryonic growth & healing processes. (Becker et al. 1962, Becker 1967, 1974, Borgens 1982, Foulds and Barker 1983, Hinkle et al. 1981, Illingworth and Barker 1980, Patel and Poo 1982).
- that by enhancing the endogenous electrical activity of the damaged tissues, the growth and/or healing processes can be stimulated or enhanced. (Brighton et al. 1981, Brown et al. 1988, Carley and Wainapel 1985, Kincaid 1989, Kloth and Feedar 1988, Reed 1988, Rowley et al. 1974, Wheeler et al. 1969).
The subject of endogenous bioelectricity is somewhat larger than can be detailed here, though what follows may provide a useful overview.
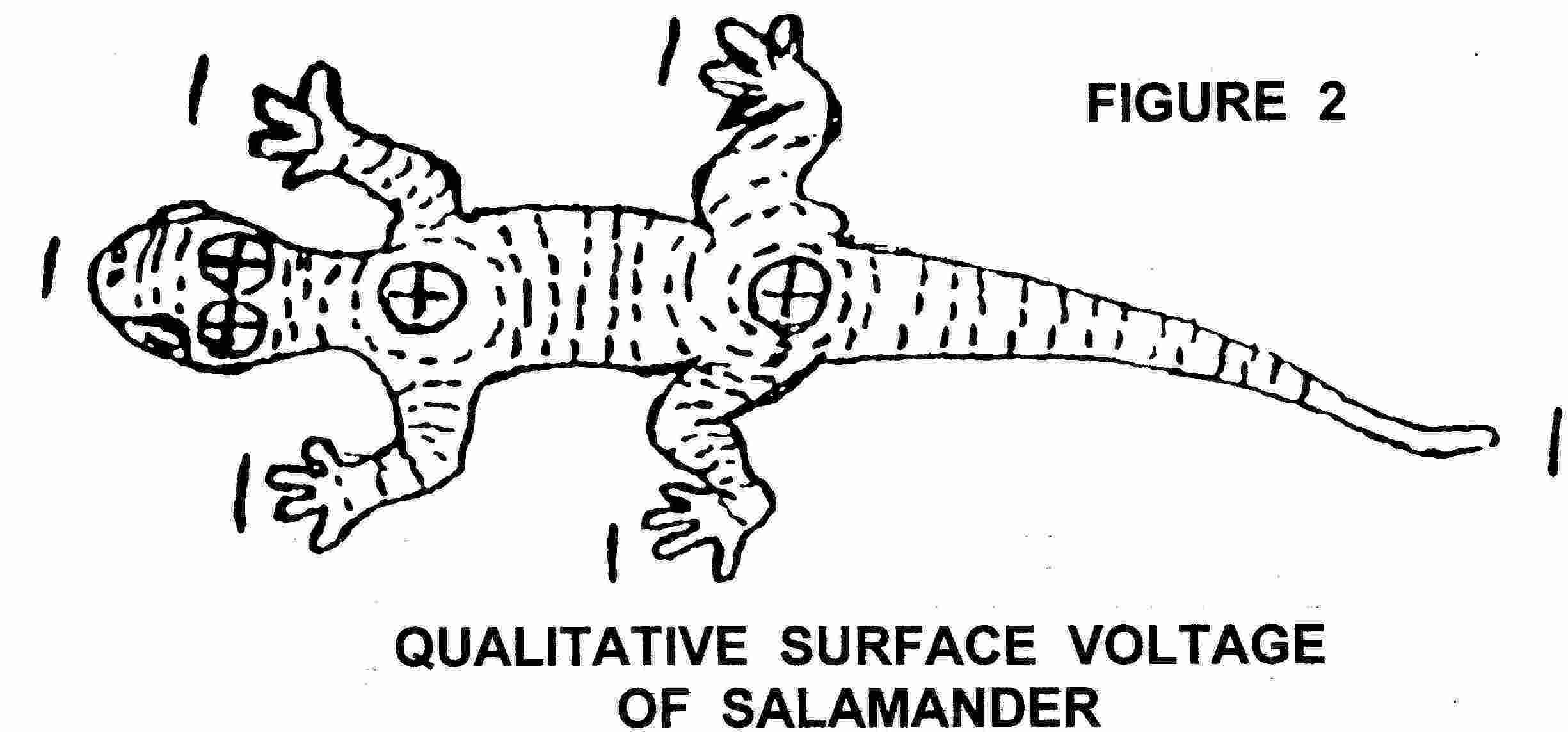
Tissue batteries are found in bone, skin and muscle and nerve, and probably other musculoskeletal tissue. These tissues seem to produce a potential difference between various parts of the tissue, and this potential is different in the injured and non injured situations. Bone exhibits piezoelectric potentials, streaming potentials and steady potentials (Fukada 1984, Pollack et al. 1984, Friedenberg et al. 1973, Borgens 1984, Chakkalakal et al. 1988). In the skin, there are a wealth of bioelectric phenomena, but particularly in this context, Skin Potential Levels, Responses to psychological stimulation and changes associated with injury and pathology (Barker et al. 1982, Edelberg 1968, Foulds and Barker 1983, Christie and Venables 1971, Millington and Wilkinson 1983, Vanable 1989). Similar potentials have been shown in muscle (Betz et al. 1984, 1986) and collagenous based tissues (Anderson and Eriksson 1968, Athenstaedt 1970).

Growing and Developing Tissues exhibit numerous very interesting bioelectric activities. Amphibian limb regeneration studies (Borgens 1982, Becker 1961, Sisken 1983), mammalian partial limb regeneration (Neufeld 1989, Becker 1972) and rabbit ear regeneration (Chang and Snellen 1982, Goss 1981) have been reasonably well investigated. Fingertip amputation and regeneration in children provided the focus for an interesting study (Illingworth and Barker 1980). In addition, there has been considerable work into electric effects in embryology and morphogenesis (Robinson 1989, Jaffe 1981,1986, Jaffe and Nuccitelli 1977, Nuccitelli and Erickson 1983)
Injured Tissues are reported to be electrically active, and this activity appears to be more than just an epiphenomenon. The exact origin of the injury potential remains debatable (Nordenstrom 1983, Thakor and Webster 1978, Becker 1967, 1974). Tissues which have demonstrable electrical changes on injury include Skin (Barker et al. 1982, Foulds and Barker 1983, Jaffe and Vanable 1984, Vanable 1989), Bone (Friedenberg et al. 1973, Lokietek et al. 1974, Chakkalakal et al. 1988, Borgens 1984), Muscle (Lokietek et al. 1974, Betz et al. 1984), Nerve (Shibib et al. 1988a,b, Borgens and McCaig 1989), Blood vessels (Sawyer et al. 1953) and several others. Ulcers and other skin wounds appear to have electrical links (Carley and Wainapel 1985, Rowley 1985, Griffin et al. 1991, Kloth and Feedar 1988, Reed 1988).
Cells which are electrically responsive include FIBROBLASTS ( Vanable 1989, Weiss et al. 1990, Erickson and Nuccitelli 1984, Dunn 1988), EPIDERMAL CELLS (Brown et al. 1988, Brown et al. 1989, Luther and Peng 1983, Robinson 1985, Vanable 1989), MACROPHAGES (Vanable 1989), NEUTROPHILS AND ERYTHROCYTES (Becker and Murray 1967), BONE AND ARTICULAR CARTILAGE (Brighton et al. 1981, Rubinacci and De Loecker 1988, Norton et al. 1977, Okihana et al. 1985), TENDON (Stanish et al. 1985), LIGAMENT (Akai et al. 1988, Frank et al. 1983).
Psychological and Emotional factors include a wide range of electrodermal activity (Edelberg 1968, Edelberg 1971, 1977, Christie 1981), links with hypnosis and sleep (Becker 1974, Leonesio and Chen 1987), electroanalgesia (Becker 1990), some voluntary control may be possible using biofeedback (Nishimura and Nagumo 1985, Volow et al. 1979), and finally, possible links with psychiatric disorders (Venables 1978, Williamson et al. 1985).
The Bioelectric Cell
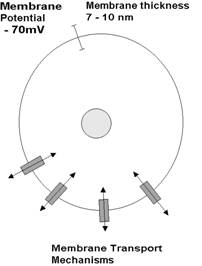
| Every living cell has a membrane potential (of about -70mV), with the inside
of the cell being negative relative to its external surface. The cell membrane
potential is strongly linked to the cell membrane transport mechanisms in that
much of the material that passes across the membrane is ionic (charged
particles), thus if the movement of charged particles changes, then it will
influence the membrane potential. Conversely, if the membrane potential
changes, it will influence the movement of ions. Relative to the size of the cell, the membrane potential is massive. The membrane is, on average 7-10 nm thick (a nanometre is a thousandth of a millionth of a metre). The equivalent voltage is somewhere in the order of 10 million volts per metre (which is reasonably impressive!). |
 |
Lightning Bolts within Cells
A new nanoscale tool reveals strong
electric fields inside cells.
|
|
|
The cell electric:
Encapsulated in a polymer shell just 30 nanometers across, voltage-sensitive
dyes (red) emit red and green light when illuminated with blue light. These
encapsulated dyes make it possible to measure electric fields inside cells. |
Using novel voltage-sensitive nanoparticles, researchers have found electric fields inside cells as strong as those produced in lightning bolts. Previously, it has only been possible to measure electric fields across cell membranes, not within the main bulk of cells. It's not clear what causes these strong fields or what they might mean. But now that it's possible to measure them, researchers hope to learn about disease states such as cancer by studying these electric fields.
University of Michigan researchers led by chemistry professor Raoul Kopelman encapsulated voltage-sensitive dyes in polymer spheres just 30 nanometers in diameter. When illuminated with blue light, the voltage-sensitive dyes emit a mixture of red and green light; the exact frequency of light emitted is influenced by the strength of local electric fields, allowing the researchers to measure those fields. Testing these nanoparticles in the internal fluid of brain-cancer cells, Kopelman found electric fields as strong as 15 million volts per meter, perhaps five times stronger than the field found in a lightning bolt.
"They have developed a tool that allows you to look at cellular changes on a very local level," says Piotr Grodzinski, director of the National Cancer Institute Alliance for Nanotechnology in Cancer. Traditional techniques for studying disease at the level of tissues average out differences between cells. Grodzinski says that many developments in cancer research over the past few years have been "more reactive," working toward developing diagnostics for catching the disease in its earlier stages and for better predicting to which drugs patients will respond. Despite how far cancer treatments have come, the way that cancer progresses at the cellular level is still not very well understood. With a better understanding, researchers hope to further improve diagnostics and personalized care. "This development represents an attempt to start using nanoscale tools to understand how disease develops," says Grodzinski.
Jerry S.H. Lee, a nanotechnology project manager also at the National Cancer Institute, says that Kopelman's research bolsters the set of nanoscale tools that scientists are developing to probe cells' physical properties, such as special microscopic probes for measuring cell stiffness. (See "The Feel of Cancer Cells.") In the past decade, researchers have improved cancer diagnosis by examining protein markers and genetic signatures. Now they're "thinking of how nanotechnology can make tools to look at additional signatures" like electric fields, says Lee.
Voltage-sensitive dyes are not new. For decades, neuroscientists have used them to measure voltages across cell membranes in studies of how nerve cells generate and respond to electrical charges. But Kopelman says that it's not possible to control the placement of these dyes in cells. They are hydrophobic and aggregate in cell membranes, so it has not been possible to use them to study the cytosol, the bulk of the interior of the cell. Kopelman also says that these dyes might be reacting with enzymes and other molecules in cells. His encapsulated dyes aren't hydrophobic and can operate anywhere in the cell, not just in membranes. Because it's possible to place his encapsulated dyes in a cell with a greater degree of control, Kopelman likens them to voltmeters. "Nano voltmeters do not perturb [the cellular] environment, and you can control where you put them," he says.
"Nanosized
Voltmeter" Enables Cellular-Wide Electric Field Mapping
* Toxicology Program and Chemistry Department, University of Michigan, Ann Arbor, Michigan
Correspondence: Address reprint requests to Raoul Kopelman, Dept. of
Chemistry, University of Michigan, 930 N. University, Ann Arbor, MI 48105.
Tel.: 734-764-7541; Fax: 734-936-2778; E-mail: kopelman@umich.edu.
Previously, all biological measurements of intracellular electric fields (E fields), using voltage dyes or patch/voltage clamps, were confined to cellular membranes, which account for <0.1% of the total cellular volume. These membrane-dependent techniques also frequently require lengthy calibration steps for each cell or cell type measured. A new 30-nm "photonic voltmeter", 1000-fold smaller than existing voltmeters, enables, to our knowledge, the first complete three-dimensional E field profiling throughout the entire volume of living cells. These nanodevices are calibrated externally and then applied for E field determinations inside any live cell or cellular compartment, with no further calibration steps. The results indicate that the E fields from the mitochondrial membranes penetrate much deeper into the cytosol than previously estimated, indicating that, electrically, the cytoplasm cannot be described as a simple homogeneous solution, as often approximated, but should rather be thought of as a complex, heterogeneous hydrogel, with distinct microdomains.
Zap
By Karl H. Schoenbach, Richard Nuccitelli, and Stephen J. Beebe
Illustration: Bryan Christie Design
Illustration: Bryan Christie Design
40 Thousand volts, four thousand amperes, and over one hundred million watts squeezed into a cubic centimeter. You’d think that would be enough to vaporize just about anything, and it certainly doesn’t seem like the kind of electricity you’d want to apply to your body. But if our research continues to succeed as it has, years from now we’ll be asking some cancer patients to do just that. And it might just save their lives.
The trick is to apply that gargantuan jolt for only a few billionths of a second. That’s so brief a time that the energy delivered is a mere 1.6 joules per cubic centimeter—barely enough to warm a thimbleful of water by a third of a degree Celsius. But these powerful, ultrashort voltage pulses do something nothing else can—harmlessly slip past a cell’s exterior to shock the vital structures within.
The effects of such pulses of power on living tissue are profound and varied. Malignant tumors—in mice, at least—can be completely wiped out, even by significantly lower power levels; new genes can be efficiently inserted into living cells in the hope of correcting genetic defects; and immune-system cells can be marshaled to fight off invading microbes.
A new field of research, bioelectrics, is emerging to study these effects, as well as the naturally occurring electric fields in biological systems. Bioelectrics relies on a curious pairing of disciplines that until now have had almost nothing to do with each other: high-voltage engineering and cell biology. In particular, the new field depends on advanced pulsed power technology. That’s the ability to switch on and off thousands of amperes of current and just as many volts in mere nanoseconds (the kind of parameters needed to detonate nuclear bombs, it so happens).
The use of high voltages and currents to manipulate structures inside cells is barely five years old, but it is a fast-growing international research endeavor. The largest R&D program at the moment is being supported by the U.S. Air Force Office of Scientific Research, in Arlington, Va. That program supports work at a new center established jointly by Old Dominion University and Eastern Virginia Medical School, where we authors are working, as well as at several other institutions in the United States, including the Massachusetts Institute of Technology, the University of Texas Health Science Center, the University of Wisconsin–Madison, and Washington University. Progress in this program has already sparked interest and some excellent science at academic institutions in Japan, China, and the state of California. And more institutions, notably in the UK, France, and the state of Missouri, are planning bioelectrics research.
It’s easy to see the attractions for biologists and for engineers. For biologists, it’s the potential scientific payoff: these strong but exceedingly brief electric fields act as a kind of electrical probe, letting scientists prod key structures inside cells—making the cells expel certain vital chemicals or begin the production of others—with the aim of understanding basic biological processes. For engineers, it’s the opportunity to forge an important new application of pulsed power technology, which even 10 years ago was seldom used outside the military.
The most promising and practical result so far has been our recent discovery that certain pulsed electric fields can wipe out skin tumors in mice. Melanoma, the skin cancer we’ve worked with, is an extremely aggressive disease that kills about 8000 people a year in the United States alone. A few hundred pulses totaling just 120 microseconds of treatment shrank tumors in mice by 90 percent. A second treatment, days later, destroyed the tumors completely.
Biomedical science is, of course, littered with cancer cures that work in mice but fail or are impractical in humans. And it will be many years before we know if bioelectrics will even be worth testing in humans. Nevertheless, even at this early stage, bioelectrics seems to offer a totally new therapeutic avenue—one that could lead to a therapy free of the debilitating side effects of chemotherapy drugs and the tissue damage of radiation.
To understand what happens when a cell is hit with tens of thousands of volts, and why it may help cure disease, you have to know something about cells themselves. At its simplest, a cell is a pocket of water containing a bunch of small functional units called organelles, which are bounded by oily membranes. These organelles are the cell’s version of internal organs: they perform the functions that keep the cell alive, just as the brain, kidneys, and lungs, among other organs, keep the body alive.
Cells do the things they need to do—contract if they are muscle cells, sense light if they are retinal cells, transport oxygen if they are blood cells—because they produce proteins with specialized functions. The creation of proteins begins in the nucleus, the cell’s most prominent and arguably most important organelle. It houses the cell’s fantastically complex genetic programming apparatus, which lets the cell repair itself and tells it how and when to reproduce, what to do when it detects a particular hormone, and how and when to die. Errors in these genetic programs go to the heart of most of the diseases suffered by humankind. These errors can predispose a person to heart disease, cancer, schizophrenia, and countless other maladies.
The programs are written into your genetic code. This code exists physically as a set of 23 pairs of chromosomes that reside in the nucleus. Each chromosome is a rod-shaped or threadlike structure of deoxyribonucleic acid, or DNA, made up of a sequence of four chemical building blocks. The sequence of these building blocks—there are tens of millions of them on a single chromosome—is the code, and the “words” of this code are genes. In effect, genes are segments of a chromosome’s DNA. They are groups of many thousands of building blocks that encode a specific protein, with each chromosome containing thousands of genes.
These genes are the blueprints for the proteins that determine whether you have brown or blue eyes, whether your hair is straight or curly, whether you are tall or short, and whether you are likely ever to suffer from depression, schizophrenia, or cancer. That’s why gaining control of what goes on inside the nucleus—which genetic programs are turned on or off and when—has been a primary goal in biomedical science practically since the discovery of the structure of DNA about 50 years ago. It is the object of the long-standing, multimillion-dollar research endeavor called gene therapy, which after decades of work in some of the world’s foremost laboratories has had mixed results.
Basically, our work with nanoseconds-long, high-voltage pulses offers a way to gain access to the cell’s organelles, including its nucleus—something that has historically bedeviled biomedical scientists. Remember that the cell and its organelles are bound by membranes. The main component of these 5-nanometer-thick boundaries is called a phospholipid bilayer. It is an oily barrier that blocks the flow of water and ions and therefore also blocks the flow of electric current.
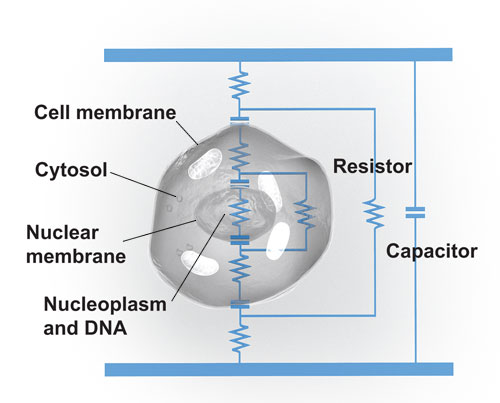
However, the membranes are also studded with proteins, some of which form nanometer-scale channels designed to allow specific ions to flow in a direction useful to the cell. In a way, a cell’s membranes are like leaky capacitors. (Some, such as the one surrounding the nucleus, leak more than others.) To extend this analogy, the briny fluid within the membranes, the cytosol, is conductive and can be thought of as a resistor [see illustration]
|
|
Cellular Circuit: A cell can be thought of as a circuit made up of capacitors and resistors. Its membrane and those of its organelles, such as the nucleus, act like capacitors. The briny liquid encased within the membranes, the cytosol and nucleoplasm, is conductive and so can be modeled as resistors.
Illustration: Bryan Christie Design
Now consider what happens when you apply a pulse to the cell. In general, there are four important characteristics that determine the precise effects. These are how fast the pulse comes on, or its rise time; how long the pulse lasts; how many pulses there are; and, of course, how great the peak voltage is. Different values for each produce a range of effects, but it’s a very fast rise time that makes it possible to electrically manipulate organelles.
To see why rise time is critical, imagine a long voltage pulse applied to the cell that comes on rather slowly, in milliseconds, say. This slow-rising pulse will set up an electric field across the cell membrane. In response, ions dissolved in the cell’s cytosol will stream to the cell membrane, charging it up to counteract the applied field. Because the voltage is rising rather slowly, the ions have enough time to accumulate at the cell membrane and cancel out the electric field, thereby shielding internal structures, such as the nucleus, from the voltage.
Now, as with any capacitor, if too much charge gathers at the cell membrane, the electric field there breaks the membrane down. In a cell, this means large holes, or pores, form in the membrane and allow ions to pour across, short-circuiting the cell. This effect is called electroporation, appropriately enough, and it is generally reversible and even useful. Scientists hoping to kill tumors more efficiently, use electroporation experimentally, for instance, to increase the amount of chemotherapy drugs that tumors take up. In fact, San Diego–based Inovio Biomedical Corp. is in the late stages of clinical tests on such a cancer treatment for tumors of the head and neck.
To manipulate a cell’s internal structures, we want instead to generate a strong electric field inside the cell, and do it before too much charge has accumulated at the cell membrane and turned it into Swiss cheese. Take the case of a brief pulse with a fast rise time, reaching its full force in a matter of nanoseconds. With so brief a rise time, not enough ions will have time to reach the cell membrane to counteract the sudden electric field, so the nucleus and other organelles will feel the field’s full effect.
For pulses with a fast rise time, then, the electric field charges up the membranes of both the cell and its organelles. Generally, the cell’s plasma membrane doesn’t fully charge to the point where large pores form in it until it’s been exposed to at least a microsecond and typically tens of microseconds of voltage. Because the organelles are much smaller than the cell itself, however, they reach their maximum charge much more quickly. Ending the pulse after the organelles are charged up, within a few hundred nanoseconds but before large pores appear in the cell’s own membrane, lets you focus the electric field’s effects on the organelles, such as the nucleus, while leaving the cell membrane relatively untouched. That, in turn, lets you do the complex and varied things medical science is interested in, such as killing tumor cells or triggering an immune system response.
This new ability to electrically tweak a cell’s insides would not exist without pulsed power technology: generating, measuring, and using extremely high-power electric pulses. Developed initially to power radar in World War II, pulsed power technology now drives X-ray imagers, particle accelerators, and nuclear weapons, to name a few applications.
The kinds of pulses that work best in bioelectrics are simple rectangular waves. There are a few ways to make such a pulse. The simplest is to discharge a capacitor. Provided that the time it takes the capacitor to discharge is long in comparison to the length of the pulse, you get a roughly rectangular pulse. The problem is that the pulse length is determined by the closing and opening of a switch. And no high-voltage mechanical switch can open and close in the few nanoseconds we need.
Certain types of transistors can do the trick, but they can switch only 1 kilovolt or less, and we usually need 10 kilovolts or more. Switches that can handle that kind of voltage can reliably close in just nanoseconds, but they can’t open so quickly.
What’s needed is a way to separate the length of the pulse from the speed of the switch. A transmission line pulse generator does just that. In electric power, transmission lines are generally paired conductors, such as coaxial cables, that are long in comparison to the wavelength of the signal they carry. In particular, we make use of transmission line generators in a Blumlein configuration, named for the British stereo recording and radar pioneer Alan Blumlein.
Picture two long rectangular conductors sandwiching a thin layer of insulation “Power Pulse” One conductor is divided into two pieces of equal length, and the load—in our case, a small tube of cells or a patch of tumor-riddled skin—is placed between them. The other conductor is charged up because it’s connected at one end to a high voltage. And the bisected conductor is grounded at the same end.
Illustration: Bryan Christie Design

A Blumlein generator produces brief high-voltage pulses when electromagnetic waves change polarity and collide.
Closing a switch connects the two conductors, discharging the device and setting up waves of voltage that rocket along it [see bioelectric researcher Juergen F. Kolb's animated clip of the waves in the online version of this article at http://www.spectrum.ieee.org/blumlein These waves travel in a way not unlike a wave that you’d set up on a length of rope by holding one end and snapping it.
When the switch closes, waves travel both toward and away from the load. For those initially traveling toward the load, some portion reflects off it, and the rest transmits right across it. For those waves traveling away from the load—including the portions that have now transmitted across—what happens depends on which end of the device they encounter.
Taking the rope analogy again, note that if you send a wave down a rope, the wave will reflect off the end and head back toward you. If the end is hanging loose, the reflection will be of the same phase as the initial wave. That is, if the voltage change was positive, the reflection will be, too. But secure the loose end and the wave will invert when it reflects. The unsecured rope is analogous to the end of the transmission line opposite the switch. The secured end, on the other hand, is like the end at the closed switch.
The voltage pulse comes about when the inverted reflection and the noninverted reflection crash into each other at the load. The pulse ends when the trailing edge of each wave has completed its trip down the transmission line to the load. Therefore, it is not necessary to open a switch to terminate the pulse; it simply ends abruptly when there is no energy left in the line. What’s more, you can easily adjust the duration of the pulse by either adding or subtracting length from the transmission line.
So what happens to a cell when you zap its innards with so much power? We’re still working out the biological details, but experiments using cancer cells suspended in liquid or even growing as tumors in mice have yielded a good deal of insight.
In our most recently reported experiments, we injected melanoma cancer cells under the skin of 120 mice and allowed tumors to form [see photo, “
|
|
Diminished: A skin tumor in a mouse before [top] and 16 days after [bottom] treatmentwith nanoseconds-long pulses of voltage.
Images: Frank Reidy Research Center for Bioelectrics
” We then used a Blumlein pulse generator to subject
the tumors to electric field pulses 300 nanoseconds long—too short to cause
classical electroporation—that reached 90 percent of
their peak of 40 kilovolts per centimeter in just 30 ns. We hit the tumors with
a total of 400 pulses, one every other second. Over the course of two weeks,
the tumors shrank by 90 percent. Then they began to grow again. But in a few
experiments, we subjected the tumors to subsequent sets of pulses, and they
were destroyed completely and did not grow back.
We believe our ultrashort electric pulses killed the tumor cells by kick-starting a cellular phenomenon called apoptosis, but proving that theory beyond a doubt is difficult. Apoptosis is also called cell suicide or programmed cell death. In apoptosis the cell disassembles itself in an orderly fashion in minutes or hours, leaving behind only fragments useful as recycling material for the body. It is a process that allows the removal of cells that are no longer needed by the organism or of cells that pose a threat to it. As part of the apoptosis system, cells can sense if they are too badly damaged to reproduce correctly. Almost by definition, in cancers, the apoptosis system is off-line, allowing a dangerously aberrant cell not only to survive but also to multiply.
We saw two nearly immediate effects of the pulses in the mouse tumors that could indicate apoptosis. First, within just a few minutes, the tumor cell nuclei had shrunk to half their original sizes, suggesting that the electric field had either directly or indirectly damaged the cell’s DNA.
Also, separate experiments done on cells in a liquid suspension showed that similar pulses resulted in broken DNA and that genetic programs involved in DNA repair became more active in the pulses’ aftermath. DNA damage can trigger apoptosis, but such damage also occurs during apoptosis. However, a classic experiment to prove that apoptosis is in progress, measuring the amount of a chemical called caspase in cells, showed no change and no apoptosis.
We think that’s because of the second immediate effect we observed: within minutes of treatment, blood stopped flowing to the tumor. It takes energy for a cell to kill itself—in other words, apoptosis can’t happen without a steady supply of nutrients and oxygen from the blood. Though we don’t know the exact reason blood stops flowing, stopping the flow is clearly helpful in destroying tumors. Malignant tumors can grow to dangerous proportions only because they have the ability to trick the body into growing new blood vessels to feed them. Developing drugs to starve tumors by disrupting their blood supply and their ability to build a new supply is a major goal of many pharmaceutical firms. And it appears that disrupting the blood supply is something that nanoseconds-long pulsed electric fields can do.
A key measure of how useful a cancer treatment might be is if it is more harmful to tumors than to normal tissue. When we shocked vials containing both cancer cells and normal cells, the pulses killed only the cancer cells. However, in the mouse experiments, our pulses did some damage to healthy skin surrounding the mouse tumors. But this blackening was temporary, and within a couple of weeks, the skin had healed. Minor tissue damage is common in cancer therapies. In fact, most treatments, such as chemotherapy, are damaging to tumors and healthy tissue alike, but they rely on the fact that healthy tissue has working genetic programs that allow it to survive the chemical attack and tumors do not.
We are probably years away from performing a similar test of ultrashort high-power pulses on human cancer patients. But even if those tests are successful, there will be many hurdles to overcome for nanosecond pulsed electric fields to become a viable treatment in the clinic. For one thing, we must be able to deliver gigawatts of power accurately to sites deep within the body—not just at the skin surface where we can pinch the tumor between two parallel plates or poke it with pin electrodes. And we must be able to do so with little or no harm to the surrounding healthy tissue.
So this summer we are working with antenna expert Carl E. Baum, at the University of New Mexico, in Albuquerque, to build a device to let us beam the pulses at cells deeper inside the body. When pressed against the skin, such a pulse generator’s half-ellipsoid antenna should focus an electric field pulse to a small volume several centimeters inside the body. The antenna is only at the modeling stage, but using our existing laboratory equipment we have begun to examine what sorts of pulses it would create and what those pulses would do to living cells.
For the time being, though, and notwithstanding the fact that we’ve made a lot of progress observing the effect of this high-voltage treatment on tumors in mice, we know far too little about it now to move on to experiments in humans. It’s important to keep in mind that the majority of new therapies that show promise in the lab never develop into approved treatments. We hope nanosecond pulses will, but the road ahead will be twisty and difficult.
Cancer cells are just one target of ultrashort pulsed electric fields. By lowering the power and altering their target, for example, we can also use the pulses in gene therapy. For instance, in proof-of-principle experiments, we used the pulses to insert new genes into chromosomes in the nuclei of cells—one of the key challenges of gene therapy.
For various reasons, the enormous potential of gene therapy has largely eluded medical researchers. Basically, the techniques have proven difficult for physicians to execute and dangerous for patients subjected to them. A prominent example of gene therapy in humans was a trial in Europe in the 1990s to treat severe combined immune deficiency syndrome. Commonly called “bubble boy” syndrome, the disease is caused by an inherited defect in a single gene, which cripples the body’s defense against infection.
To combat the disease, doctors introduced a corrected copy of the gene into the nuclei of the children’s immune-cell-generating tissue. Encouragingly, the therapy defeated the disease, but unfortunately, three of the first 11 patients developed leukemia, caused by the way the new gene inserted itself into their existing DNA. Despite the setbacks, medical scientists have not given up on gene therapy for bubble-boy syndrome and are also trying it out for nerve damage from diabetes, heart failure, hemophilia, and a host of other diseases.
Another reason to insert new genes into people is to immunize them against a particular disease. Ordinary vaccines provide immunity because they are made up of crippled or dead versions of a disease-causing microbe. Exposure to a neutered version of the microbe enables our immune systems to recognize the chemical characteristics of the weakened microbe and to mount a fast, effective defense against the real version. However, the vaccine must be refrigerated, and if the microbe is not weakened enough—and this only very rarely occurs—it can cause rather than protect against the disease.
Partly because of these drawbacks, researchers have become intrigued with the idea of injecting a person with the DNA that codes for one of the infectious bug’s proteins. Some of the person’s own cells take up the DNA, produce the protein, and trigger the immune system to learn to recognize and defend against any microbe carrying that protein.
Among the chief technical difficulties with these DNA vaccines, as well as with gene therapies, is getting the DNA into cells. Simply injecting a dollop of DNA into someone is not good enough, because the cell membrane is such a strong barrier against DNA. One popular solution is to actually genetically engineer the DNA into a virus. Viruses infect us by “sneaking” their genetic material through the cell membrane and tricking the cell into copying it. So scientists have sought to include the DNA they want into harmless viruses, with which they then infect the patient in the hopes that the virus will deliver the new gene to the place it needs to go.
The problem is that the virus can stitch the new gene into a bad spot in the cell’s own DNA, disrupting an important chemical program and causing disease, as happened when the immune-deficient children developed leukemia. Or the virus itself can cause a runaway immune system reaction that can kill the patient, as seems to have happened in a gene therapy trial several years ago at the University of Pennsylvania, in Philadelphia.
Pulsed electricity may offer a safer solution. First we can use strong, but rather long-lasting, electric fields to induce electroporation, the state we mentioned earlier in which the cell’s outer membrane temporarily becomes porous. This works, to a point, because although the new DNA can now enter the cell, it must still get past the nucleus’s membrane for the cell to decode it.
Because the ultrashort pulses we’ve worked with appear to affect subcellular membranes, such as the double membrane that bounds the nucleus, we figured they might help genes make it through that last step of their journey by opening pores in the nuclear membrane. As a test, we tried to insert a certain gene from a jellyfish into bone marrow cells in a test tube. If this gene makes it into the nucleus and is decoded, it produces a protein that glows green.
By itself, electroporation improved the amount of the gene that was taken into the cells’ nuclei by 260 percent, as measured by the number of cells glowing and the strength of the green glow. But following electroporation with a nanoseconds-long pulse aimed at opening the cells’ nuclei increased gene uptake by a whopping 900 percent—potentially enough to improve the efficiency and safety of gene therapies or DNA vaccinations.
The list of effects that scientists have achieved using nanoseconds-long pulses is growing rapidly, though their actual use as a medical treatment is still years away. For example, brief pulses cause platelets, cellular fragments in the blood, to begin the complicated cascade of steps needed to form clots. Though the experiments were performed in a test tube rather than on a human being, we hope the effect might one day be used in healing wounds.
In other research, E. Stephen Buescher, a professor of pediatrics at Eastern Virginia Medical School, did a fascinating set of experiments with white blood cells that also might ultimately help heal wounds. In it, he observed the effect of ultrashort pulses on the release of calcium inside cells from internal stores. Calcium acts as a kind of signal transducer in many cells, translating an external signal such as a hormone into some cellular action, such as manufacturing a protein.
In a type of white blood cell whose purpose is to seek out foreign material and digest it, for example, the release of calcium allows the cell to follow an invader’s chemical trail. When Buescher subjected these cells, called leukocytes, to nanoseconds-long, 12-kV/cm electric fields, the cells immediately, but briefly, spilled calcium from their internal stores into their own cytosol. In experiments where the cells were actively crawling over a microscope slide, hot on the simulated trail of an invader, pulsing stopped them in their tracks and then sent them marching off in the direction of the electric field. One day doctors might use such an effect to recruit immune cells to the site of an infection.
The list of cells and the effects of pulsed power on them goes on and will only get longer as more laboratories begin work in bioelectrics. Scientists at Kumamoto University, in Japan, for example, are studying the subcellular effects of high-power RF pulses. Those at Karlsruhe University, in Germany, are testing nanopulses for killing bacteria. And researchers at the University of Southern California are studying how the pulses cause dying cells to signal other cells to consume them. Whether or not pulsed power becomes a cancer treatment, a gene therapy technology, or an infection fighter, ultrashort electric fields have already proved a powerful research tool. And the mark they ultimately make on medicine may be in allowing scientists unprecedented access to the internal workings of cells.
Still, we hope for more practical—and potentially lucrative—possibilities. While treatments for cancer and genetic diseases would be revolutionary, somewhat more prosaic applications are in the offing. We at Old Dominion University have recently used nanosecond pulsed electric fields to destroy fat cells. Think of it as electric liposuction. Hey, if it helps pay for the research needed to fight dread diseases, we’re all for it.
About the Authors
Karl H. Schoenbach, an IEEE Fellow, holds the Frank Batten Endowed Chair in Bioelectric Engineering at Old Dominion University, in Norfolk, Va. There he directs the Frank Reidy Research Center for Bioelectrics.
Richard Nuccitelli is a biophysicist at Frank Reidy who has studied the role of ion currents and ion concentration changes in the regulation of cell physiology for 30 years. He was the lead investigator on the melanoma project.
Steven J. Beebe is a faculty member in the department of physiological sciences and pediatrics at Eastern Virginia Medical School, in Norfolk, and is on the staff at Frank Reidy. He has studied mechanisms for signal transduction and apoptosis regulation for decades.
Acknowledgments
The authors would like to thank Peter F. Blackmore, E. Stephen Buescher, Ravindra P. Joshi, Juergen F. Kolb, and R. James Swanson.
To Probe Further
Proceedings of the IEEE devoted its July 2004 issue to pulsed power technology and its applications. The issue includes a more detailed look at bioelectrics: “Ultrashort Electrical Pulses Open a New Gateway Into Biological Cells,” by Karl H. Schoenbach et al., pp. 1122–37.
For more on the effects of nanosecond pulses on cell biology, see “Nanosecond Pulsed Electric Fields Modulate Cell Function Through Intracellular Signal Transduction Mechanisms,” by Stephen J. Beebe et al., Physiological Measurements, Vol. 25, 2004, pp. 1077–93.
For the latest on the authors’ experiments on melanoma, see “Nanosecond Pulsed Electric Fields Cause Melanomas to Self-destruct,” by Richard Nuccitelli et al., Biochemical and Biophysical Research Communications, 5 May 2006, pp. 351–60.