Business Week March 2005
Rewiring
The Body
First came pacemakers. Now exotic implants are bringing new hope to
victims of epilepsy, paralysis, depression, and other diseases
The potential is great.
Unlike most drugs, these implants produce few side effects. The devices also
are aimed at prevalent diseases that can't always be treated with drugs. As a
result, medical-products executives and their surgeon partners predict that
such implants could one day become as common as cardiac devices, which are
currently helping 2 million Americans.
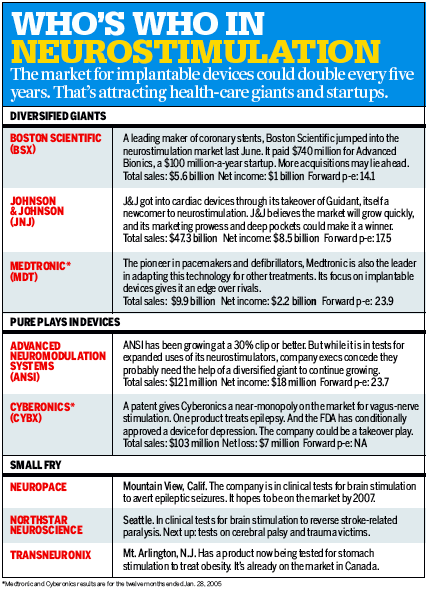
Little wonder, then, that some of the biggest names in health care are in a
scramble to get into the market. Most recently, in December, Johnson &
Johnson (JNJ ) bought implant-maker Guidant Corp. (GDT
) for $23.9 billion. "Any organ that a
nerve can influence -- and that's every organ in the body -- can be affected
using this technology," says Dr.
Ali R. Rezai, who is director of functional neurosurgery at the
The use of implantable mini-generators is more widespread than you probably
think. Already, 190,000 patients are wearing electrodes in their heads
to control Parkinson's disease tremors or spinal-cord stimulators to relieve
pain or prevent urinary incontinence. Some 30,000 have wires threaded to the
vagus nerve in the neck to treat epilepsy, while 60,000 have microtransmitters in the inner ear enabling them to hear.
These numbers are likely to grow -- and quickly. One of the most promising
devices is a $15,000 neurostimulator for chronic depression from Cyberonics
Inc. (CYBX ), which the Food & Drug
Administration conditionally approved on Feb. 2.
 Candy Bradshaw
can testify to the power of neurostimulation. She had a gastric pacemaker
implanted in her abdomen in 1999 at
Candy Bradshaw
can testify to the power of neurostimulation. She had a gastric pacemaker
implanted in her abdomen in 1999 at
At
As neurostimulators get even smaller and their microchips more powerful,
researchers foresee new uses for these implants. Advanced
Bionics Corp., a startup that Boston Scientific Corp
(BSX
). acquired in 2004, is testing a rechargeable
device so tiny that it can be injected almost anywhere in the body to treat
pain or muscle dysfunction. Implants also could act as sensors, telling a
miniature pump when to deliver a drug or customized protein to a precise
location in the body. "The body is on fire with electricity," says
Dr. Stephen N. Oesterle, chief medical officer at
Medtronic Inc (MDT )., the No. 1
maker of implantable electrical devices. "If you start with that concept,
then all you need is imagination."
The neuromodulation market is potentially enormous. There are up to 3 million
Americans with chronic migraines and 4 million with depression who do not
respond to drugs. The number of morbidly obese American adults is also
estimated at 4 million. An additional 5 million Americans have been crippled to
some degree by stroke, and the number grows by about 750,000 each year. Most of
these people won't rush out and have surgery. But if only a fraction get an
implant, executives at medical-device companies project that overall sales of noncardiac pulse generators should balloon from $1.6
billion today to $10 billion in 10 to 15 years, depending on how quickly
the FDA approves new uses. "Ultimately," says Todd K. Whitehurst,
vice-president for emerging indications at Advanced Bionics in
The returns for investors may also be substantial. Today, most neurostimulators
don't make money because years of research and development and marketing
outlays overwhelm what are, in the beginning, only trickling revenue streams.
Still, Advanced Neuromodulation Systems Inc. (ANSI
), of
As sales grow, device makers will be able to spread their expenses over a wider
base and become more efficient manufacturers. If the FDA approves their new
treatments, says Jan D. Wald, a medical-device
analyst at A.G. Edwards & Sons Inc. (AGE
) in
Over time, however, these devices may restore more than lives; they could save
money, too. In a comprehensive review of spinal-cord stimulation, a doctor and
an economist at
Neurostimulation has another selling point: Because the implants alter tissue
only at their points of contact, side effects are generally negligible. Most
say they can't sense the stimulation at all. Contrast that with the most common
drug treatment, Dilantin, which can cause dizziness
and nausea and can lead to liver damage. "Think of the device as a smart bomb“says Advanced Neuromodulation (ANSI
) CEO Christopher G. Chavez.
Medical-device executives and surgeons point out that today's implants are not
generally intended to be a first-line treatment. Someone with heart trouble,
for instance, would start off on a cholesterol-reducing drug and a stricter
diet before getting outfitted with an implantable defibrillator. The same goes
for neurostimulators, which are meant for patients with illnesses or
disabilities for which there are no other treatments. People like Judith Walsh
of
Last February, Walsh began electrical-stimulation therapy. In a clinical study
sponsored by Northstar Neuroscience Inc., doctors at
Today, Walsh can make a peanut-butter and jelly sandwich and grip the steering
wheel of her car with her left hand. More gratifying, she says, she can feed
and dress her five-month-old granddaughter, Emma, things she couldn't do with
her three older grandchildren when they were babies. "It's hard, as a
grandmother, not to be able to hold the grandchildren -- and now I'm able to do
that," she says. "It's the thrill of my life." Executives at Northstar, a
On Feb. 2, the FDA cleared Cyberonics' vagus-nerve stimulator for chronic
depression, pending some clarification on the labelling of the device. Chairman
and CEO Robert P. "Skip" Cummins says
Cyberonics analyzed results from 240 people with long-term depression after two
years of neurostimulation. All of the subjects had failed to respond to drugs.
The analysis found that half the patients were markedly better, with 18%
reporting they were no longer depressed. With the FDA's go-ahead, Cummins says,
Cyberonics will begin pilot studies on Alzheimer's disease, headache, anxiety
disorders, and bulimia. Medtronic also may be closing in on a number of new
therapies. Its products are in clinical tests to pulse the thalamus to treat
epilepsy; another region of the deep brain to treat migraines, depression, and
obsessive-compulsive disorder; the hypoglossal nerve in the neck to treat sleep
apnea; the sacral nerve to treat bowel disorders; and the stomach to treat
obesity. Medtronic may have a deep-brain treatment for epilepsy in two or three
years.
New treatments may become feasible as device sizes shrink and rechargeable
batteries evolve. Advanced Bionics, for example, has developed a rechargeable
implant that is about the size of an ink tube from a ballpoint pen cut to a
one-inch length. Its first use, already permitted in
The leading cardiac-device makers are packing their newest implants with enough
computing power to sense the environment around them and alter a patient's
treatment as needed. A next step would be to link sensor-laden neurostimulators
to miniature drug pumps. In this way, a patient could be dosed exactly when
needed and at the precise site where the medication is most effective.
Researchers say this could reduce dosages by a thousandfold
and avert side effects. Such systems would also enable a patient to be treated
with bioengineered drugs and proteins too large to be absorbed by swallowing a
pill. The combined therapy seems most promising in the brain, where many
disorders might be tackled with protein drugs complemented by electrical
pulses.
As these new therapies move closer to reality, the medical-products companies
are putting down their markers. Last June, Boston Scientific
paid $740 million in cash to acquire Advanced Bionics. Boston Scientific also
holds a 14% stake in Cyberonics. Then in December came J&J's megadeal with Guidant. Although Guidant does not have any
neurostimulators in clinical trials, the
